Pfizer and BioNTech explained they have initiated a rolling submission of knowledge to the US Foodstuff and Drug Administration immediately after a ask for from the agency. They expect to complete the EUA submission in the coming days and say they will also post medical trial information to the European Medications Agency and other businesses about the world.
Safety and usefulness are essential, explained Dr. Paul Offit, a member of the committee and director of the Vaccine Instruction Centre at Children’s Clinic of Philadelphia.
“The self-assurance of the American general public is dependent on that, that you you might be recommending a little something that you would give to your own little ones,” Offit instructed CNN. “It all relies upon on the knowledge. The facts will tell us just how very good these are. There should be a strong basic safety profile and a sturdy efficacy profile and immunogenicity profile. And if that is genuine, velocity doesn’t really issue, as prolonged as they have those people data.”
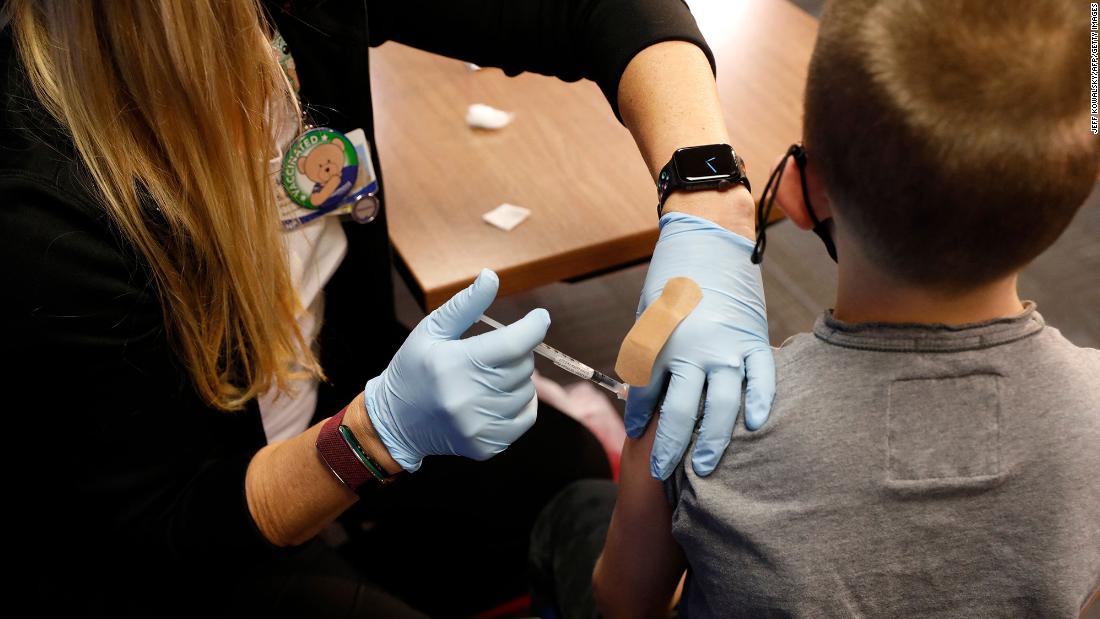
The Pfizer/BioNTech vaccine is now authorized for use in men and women as youthful as 5 and would be the initially Covid-19 vaccine offered for the youngest young children.
Since the start out of the pandemic, at the very least 11.4 million little ones have tested constructive for Covid-19, the American Academy of Pediatrics described Monday, with in excess of 3.5 million situations reported in January alone. Small children made up 22.8{cfdf3f5372635aeb15fd3e2aecc7cb5d7150695e02bd72e0a44f1581164ad809} of the whole documented weekly circumstances for the week ending January 27.
“Pediatricians have viewed firsthand the concern, strain and hardship that so many family members of young youngsters have endured as they await a vaccine,” Dr. Moira Szilagyi, president of the American Academy of Pediatrics, claimed in a assertion. “We urge a clear and facts-driven method to consider this vaccine for this age group and look ahead to offering its protection to our youngest little ones.”
The corporations are continuing to take a look at a 3-dose edition of the vaccine in the youngest children.
In December, Pfizer prolonged its vaccine trial in young kids just after two child-sized doses of the vaccine did not generate the expected immunity in 2- to 5-yr-olds, even though it did so for the infants up to age 2.
The organizations explained details on a third dose given at minimum eight months following the second dose is anticipated in the coming months, which will also be submitted to the Fda.
“As hospitalizations of small children underneath 5 due to COVID-19 have soared, our mutual purpose with the Fda is to get ready for long run variant surges and supply moms and dads with an possibility to aid defend their small children from this virus,” Pfizer Chairman and CEO Albert Bourla mentioned. “Ultimately, we consider that 3 doses of the vaccine will be required for small children 6 months by 4 several years of age to reach high concentrations of security from recent and opportunity upcoming variants.
“If two doses are authorized, mother and father will have the prospect to get started a COVID-19 vaccination sequence for their little ones when awaiting likely authorization of a third dose.”
For people today 12 and more mature, the Pfizer/BioNTech vaccine dose is 30 micrograms of vaccine, and for little ones ages 5 to 11, it was stepped down to 10 micrograms. The dose for the youngest small children is even lessen: 3 micrograms.
CNN reported previously Tuesday that Pfizer was inspired to seek authorization for the two-dose vaccine by federal regulators, who hope the EUA can be granted by late February. Waiting around on data for a few doses could extend the hold out until March.
“If the intention of the vaccine is to get baseline immunity in the young ones — to reduce seriously undesirable results and you are seriously not using the vaccine as a resource to avert an infection in the 1st position — two doses could do that,” Dr. Scott Gottlieb, a previous Food and drug administration commissioner and existing Pfizer board member, stated on CBS on Sunday. “I feel that may possibly be why federal overall health officials are rethinking this.”
CNN’s Katherine Dillinger, John Bonifield, Jen Christensen and Brenda Goodman contributed to this report.





More Stories
Vitamin D supplements linked to 40{cfdf3f5372635aeb15fd3e2aecc7cb5d7150695e02bd72e0a44f1581164ad809} lower incidence
Heart-healthy habits linked to longer life without chronic conditions
Hoda Kotb Returns To TODAY Show After Handling Daughter’s Health Matter