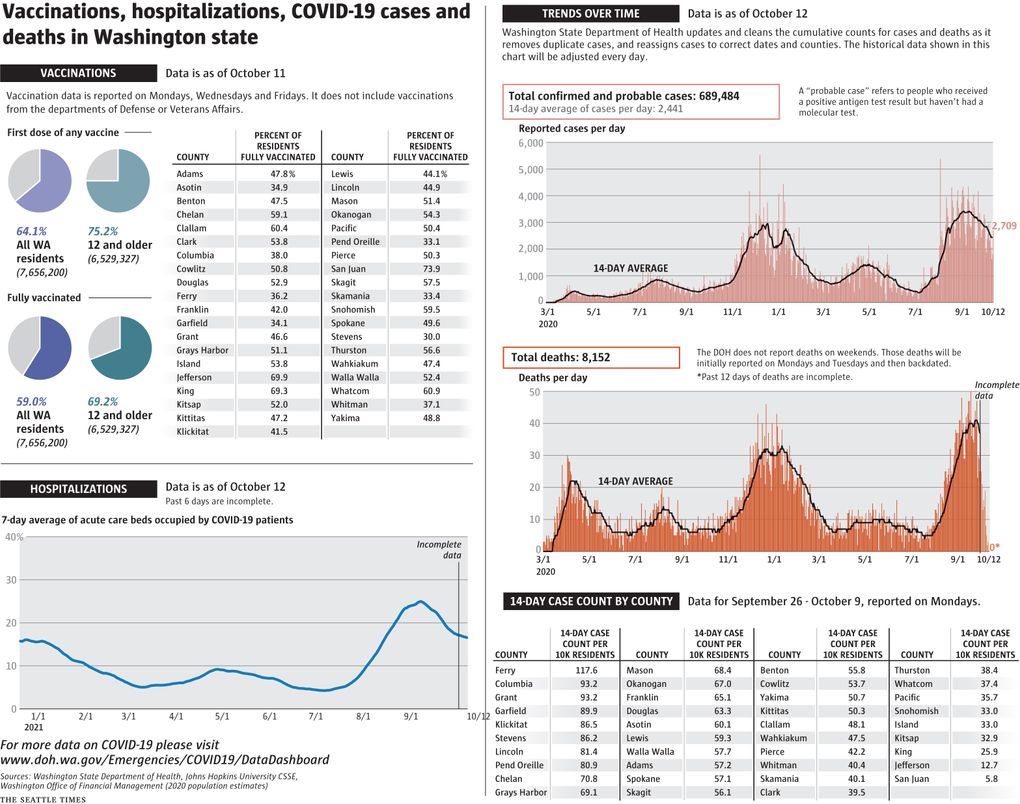
While case counts are decreasing across the state, all large events in Washington will soon require proof of vaccination or a negative test. Gov. Jay Inslee announced the new policy Thursday, which applies to indoor events with more than 1,000 people and outdoor events with more than 10,000.
Meanwhile, U.S. health advisers said Thursday that some Americans who received Moderna’s COVID-19 vaccine at least six months ago should get a half-dose booster to rev up protection against the coronavirus.
We’re updating this page with the latest news about the COVID-19 pandemic and its effects on the Seattle area, the U.S. and the world. Click here to see previous days’ live updates and all our other coronavirus coverage, and here to see how we track the daily spread across Washington.
Anchorage mask rule in effect after override of mayor’s veto
The governing body of Alaska’s largest city has overridden the mayor’s veto of an emergency order instituting a mask mandate for 60 days.
The Anchorage Assembly on Thursday overturned, on a 9-2 vote, Mayor Dave Bronson’s veto of the measure requiring masks on most everyone in indoor public spaces, Alaska Public Media reported.
The assembly had held a public hearing for a regular mask measure that drew so much opposition and had so many people wanting to comment, it stretched over multiple days.
But during a meeting Tuesday in which the proposal was not being heard, the Assembly approved an emergency ordinance putting a mask mandate in place.
Bronson, a staunch critic of COVID-19 mandates, had vetoed the ordinance Wednesday.
Read the story here.
—The Associated Press
New Mexico judge denies lab workers’ claim in vaccine fight
A New Mexico judge on Friday denied a request by dozens of scientists and others at Los Alamos National Laboratory to block a vaccine mandate, meaning workers risk being fired if they don’t comply with the lab’s afternoon deadline.
The case comes as New Mexico extends a mask mandate for indoor spaces across the state, citing persistently high levels of community spread. Nearly 263,000 COVID-19 cases have been reported in the state since the pandemic began in 2020.
While the vaccination rate among adults in New Mexico continues to hover around 71.5{cfdf3f5372635aeb15fd3e2aecc7cb5d7150695e02bd72e0a44f1581164ad809}, the rate among lab employees and contractors is much higher. The lab said last week that more than 96{cfdf3f5372635aeb15fd3e2aecc7cb5d7150695e02bd72e0a44f1581164ad809} of workers had at least one shot, but it’s not known how many have since become fully vaccinated, how many have requested exemptions or how many could end up being fired for declining the shots.
The legal challenge was backed by 114 scientists, nuclear engineers, research technicians, designers, project managers and other workers at the lab. Some are specialists and have high security clearance for the work they do, which ranges from national defense to infrastructure improvements and COVID-19 research.
The workers claim the mandate is a violation of their constitutional rights and that lab management has created a hostile work environment.
Attorneys for the lab argued in court Thursday that being vaccinated was a condition of working at Los Alamos. Lab management had announced the vaccine requirement in August.
Read the story here.
—The Associated Press
Pfizer-BioNTech ask EU agency to OK vaccine for kids 5-11
Pharmaceutical company Pfizer and biotechnology company BioNTech said Friday they have requested to have their coronavirus vaccine licensed for children ages 5 to 11 across the European Union. If EU regulators agree, it would be the first opportunity for younger children in Europe to get immunized against COVID-19.
Pfizer and BioNTech said they submitted data to the European Medicines Agency, including late-stage results from a study testing their COVID-19 vaccine in more than 2,200 children ages 6 months to 11 years. The children received a lower dose than what’s normally given to adults.
The companies said in a statement that the results showed a “strong immune response” in the children and that the vaccine was also found to be safe. There are currently no COVID-19 vaccines licensed for use in children younger than 12 in Europe or North America; the ones made by Pfizer-BioNTech and Moderna are authorized for children 12 and older in the European Union.
Read the story here.
—The Associated Press
FDA panel endorses booster shot for J&J COVID-19 vaccine
A panel of U.S. health advisers endorsed booster doses of Johnson & Johnson’s single-shot COVID-19 vaccine Friday, saying they should be offered at least two months after immunization.
J&J has asked the Food and Drug Administration for flexibility with its booster, arguing the extra dose adds important protection as early as two months after initial vaccination — but that it might work better if people wait until six months later.
The FDA’s advisory panel voted unanimously that a booster should be offered without setting a firm time. The advisers cited growing worry that recipients of J&J’s vaccination seem to be less protected than people who got two-dose Pfizer or Moderna options — and that most got that single dose many months ago.
Read the story here.
—The Associated Press
UK: 43,000 may have received false negative COVID results

British health officials said Friday that 43,000 people may have been wrongly told they don’t have the coronavirus because of problems at a private laboratory.
The U.K. Health Security Agency said the Immensa Health Clinic Ltd. lab in the central England city of Wolverhampton has been suspended from processing swabs after the false negatives.
Will Welfare, the agency’s public health incident director, said it was working “to determine the laboratory technical issues” behind the inaccurate tests.
The issue was uncovered after some people who were positive for COVID-19 when they took rapid tests went on to show up as negative on more accurate PCR tests.
The health agency said that “around 400,000 samples have been processed through the lab, the vast majority of which will have been negative results, but an estimated 43,000 people may have been given incorrect negative PCR test results,” mostly in southwest England. The incorrect results were given between Sept. 8 and Oct. 12.
Read the story here.
—Jill Lawless, The Associated Press
FDA panel takes up tough questions on J&J COVID-19 boosters
U.S. health advisers on Friday tackled who should get boosters of Johnson & Johnson’s single-shot COVID-19 vaccine and when — and whether using a competing brand for the second dose might provide better protection.
The push for boosters kicked off last month after the Food and Drug Administration authorized third doses of the Pfizer vaccine for seniors and younger adults with health problems, jobs or living conditions that place them at higher risk from the coronavirus. On Thursday, an FDA advisory panel unanimously recommended a half-dose booster of the similar Moderna vaccine for the same groups.
Friday, the same panel discussed a booster of J&J’s vaccine — but the decision is more complex. Moderna and Pfizer asked the FDA to OK boosters at least six months after immunization, but J&J proposed a sliding schedule with an extra dose as early as two months later.
Adding another twist, the experts also will discuss preliminary data from a government “mix-and-match” study that suggested J&J recipients may have a far stronger immune response if they get either a Moderna or Pfizer booster rather than a second J&J dose.
The FDA will use its advisers’ recommendations to decide whether to authorize boosters for both J&J and Moderna, likely next week, after which another government agency will rule on who should roll up their sleeves.
Read the story here.
—Lauran Neergaard and Matthew Perrone, The Associated Press
Russia breaks record again for COVID-19 deaths, infections
Russia’s daily tolls of coronavirus infections and deaths surged to another record on Friday, a quickly mounting figure that has put a severe strain on the country’s health care system.
The government’s coronavirus task force reported 32,196 new confirmed coronavirus cases and 999 deaths in the past 24 hours.
The record for daily COVID-19 deaths in Russia has been broken repeatedly over the past few weeks, as fatalities steadily approach 1,000 in a single day. It comes amid increasing infections and a reluctance by authorities to toughen restrictions that would further cripple the economy.
The government said this week that about 43 million Russians, or just about 29{cfdf3f5372635aeb15fd3e2aecc7cb5d7150695e02bd72e0a44f1581164ad809} of the country’s nearly 146 million people, are fully vaccinated. Authorities have tried to speed up the pace of vaccination with lotteries, bonuses and other incentives, but widespread vaccine skepticism and conflicting signals from officials stymied the efforts.
Despite the mounting toll, the Kremlin has also ruled out a new nationwide lockdown like the one early on in the pandemic that badly hurt the economy, eroding President Vladimir Putin’s popularity.
Read the story here.
—Vladimir Isachenkov, The Associated Press
Newly discovered bat viruses give hints to COVID’s origins

In the summer of 2020, half a year into the coronavirus pandemic, scientists traveled into the forests of northern Laos to catch bats that might harbor close cousins of the pathogen.
The fecal samples the collected turned out to contain coronaviruses, which the scientists studied in high-security biosafety labs, known as BSL-3, using specialized protective gear and air filters.
Three of the Laos coronaviruses were unusual: They carried a molecular hook on their surface that was very similar to the hook on the virus that causes COVID-19, called SARS-CoV-2. Like SARS-CoV-2, their hook allowed them to latch onto human cells.
“It is even better than early strains of SARS-CoV-2,” said Marc Eloit, a virus expert at the Pasteur Institute in Paris who led the study, referring to how well the hook on the Laos coronaviruses binds to human cells.
The findings have significant implications for the charged debate over COVID’s origins, experts say. Some people have speculated that SARS-CoV-2’s impressive ability to infect human cells could not have evolved through a natural spillover from an animal. But the new findings seem to suggest otherwise.
Read the story here.
—Carl Zimmer, The New York Times
Wyoming district where student arrested extends mask mandate

A mask-wearing mandate will continue for at least another month in a Wyoming school district where a student who wouldn’t wear a mask got arrested for allegedly refusing to leave her high school.
Grace Smith, 16, might not be involved in future confrontations at Laramie High School, however. The junior said she was withdrawing after being “bullied, discriminated against and worst of all, legitimately threatened.”
Albany County School District No. 1 trustees voted 6-1 later in the meeting to extend the district’s mask mandate for everybody inside district buildings until Nov. 12. The mask requirement had been set to expire this Friday.
Wyoming has had one of the lowest vaccination rates and highest COVID-19 rates in the U.S. but the district is among just a few in Wyoming to require masks this fall. Smith’s anti-mask stance is widespread in this conservative state, where Republican Gov. Mark Gordon has vowed not to return to mask mandates since imposing an unpopular one last winter.
Laramie — home to the University of Wyoming, which has been requiring masks in most of its buildings this fall — has been somewhat more receptive to masks than many Wyoming communities which also have had heated debates over masks this fall.
Police arrested Smith at her high school Oct. 7 after she served two consecutive, two-day suspensions for not wearing a mask.
Read the story here.
—Mead Gruver, The Associated Press
Seattle Times staff & news services







More Stories
The Layers of CMMC Compliance with a CMMC Consultant’s Aid
Fairy House: A Journey into the Magical World of Miniature Dwellings
How to promote your trips to Baku on social media