This is the 1st antiviral Covid-19 tablet approved for sick persons to just take at property, ahead of they get sick sufficient to be hospitalized.
High-hazard persons age 12 and more mature who weigh at minimum 88 lbs . and have a positive SARS-CoV-2 test are suitable for this treatment and will want to have it approved by a doctor.
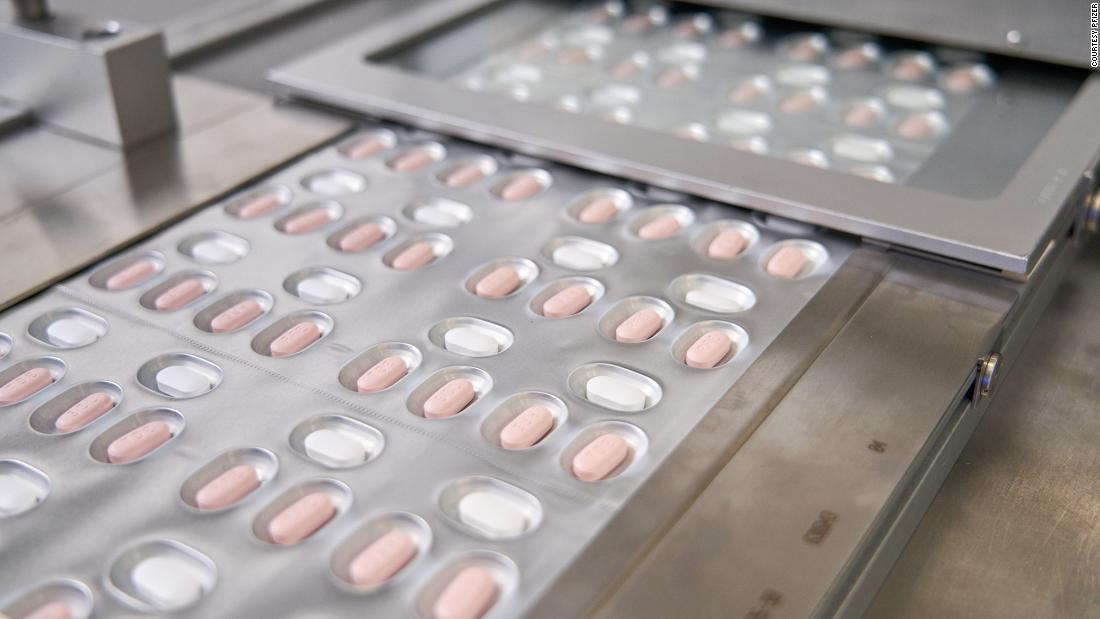
Paxlovid combines a new antiviral drug named nirmatrelvir and an older just one known as ritonavir and is administered as three tablets specified 2 times a working day for five days.
Last week, Pfizer launched updated final results that confirmed the cure slice the chance of hospitalization or dying by 89{cfdf3f5372635aeb15fd3e2aecc7cb5d7150695e02bd72e0a44f1581164ad809} if provided to high-chance adults in a couple days of their very first signs. If presented within just the initial five days of signs, the efficacy was comparable: 88{cfdf3f5372635aeb15fd3e2aecc7cb5d7150695e02bd72e0a44f1581164ad809}.
“Modern authorization of PAXLOVID represents an additional incredible case in point of how science will enable us finally defeat this pandemic, which, even two a long time in, carries on to disrupt and devastate life throughout the planet. This breakthrough treatment, which has been demonstrated to considerably lessen hospitalizations and fatalities and can be taken at home, will alter the way we treat COVID-19, and hopefully support lessen some of the important pressures facing our health care and hospital techniques,” Pfizer Chairman and CEO Albert Bourla explained in a statement. “Pfizer stands ready to start out shipping and delivery in the U.S. instantly to assistance get PAXLOVID into the arms of correct sufferers as immediately as doable.”
He called Paxlovid a “potentially impressive instrument in our combat in opposition to the virus, which includes the Omicron variant,” but pressured that acquiring vaccinated and getting a booster shot remained “the most significant resources we have to conserve lives.”
The Fda emphasized in a statement that Paxlovid is not for pre- or post-exposure avoidance of Covid-19 and “is not a substitute for vaccination in men and women for whom COVID-19 vaccination and a booster dose are proposed.”
Separately, Merck has asked for crisis use authorization for its antiviral capsule, molnupiravir. It was narrowly advisable by FDA’s advisers in a 13-10 vote at the end of November following details showed it reduce the risk of hospitalization or demise by 30{cfdf3f5372635aeb15fd3e2aecc7cb5d7150695e02bd72e0a44f1581164ad809} amid high-hazard grownups. This was lessen than an previously investigation suggesting that selection could be about 50{cfdf3f5372635aeb15fd3e2aecc7cb5d7150695e02bd72e0a44f1581164ad809}. The Food and drug administration has not introduced irrespective of whether it will authorize the treatment.
Remdesivir, offered beneath the manufacturer name Veklury, is the only antiviral accredited by Fda for treatment of Covid-19. It really is specified intravenously, not as a pill that can be taken at house.






More Stories
Heart-healthy habits linked to longer life without chronic conditions
Hoda Kotb Returns To TODAY Show After Handling Daughter’s Health Matter
Exercise 1.5 times more effective than drugs for depression, anxiety